Study of IBB and LABU
- 05/19/2020 – Moderna does smell bad from this pump and dump action.
Moderna raises eyebrows with stock offering ahead of STAT News report
- 05/18/2020 – Good news from Moderna props LABU by 10%, too bad I did not buy it or its options when it was 20% lower last week. I should response fast, especially in current high volatility conditions. I should also have to get ready to buy Moderna.
Moderna Says Initial Covid-19 Vaccine Results Are Positive
Experimental coronavirus vaccine induced immune responses in healthy volunteers who were vaccinated in a clinical study, company says
The study results—the first for the first vaccine against the new coronavirus to enter human testing—provide a positive sign about its capabilities to protect people against the new coronavirus.
Yet the results are preliminary and only for a portion of the study participants. Many vaccines fail to pass muster, even after showing positive signs in early testing.
Moderna said the results reinforce the potential for the vaccine to prevent Covid-19, the disease caused by the coronavirus.
The data suggest the vaccine, code-named mRNA-1273, “has a high probability to provide protection from Covid-19 disease in humans,” Moderna Chief Executive Stephane Bancel said on a conference call Monday.
- 05/17/2020 – short term catalyst for drugmakers
Coronavirus Vaccine Front-Runners Emerge, Rollouts Weighed
Drugmakers build capacity to make hundreds of millions of doses, while authorities discuss: Who will get it first?
Governments and drugmakers are weighing how to roll out coronavirus vaccines, including reserving the first batches for health-care workers, as several shots race to early leads.
Of more than 100 vaccines in development globally, at least eight have started testing in humans, including candidates from Moderna Inc. and Pfizer Inc. At the same time, pharmaceutical giants like Johnson & Johnson, AstraZeneca PLC and Sanofi SA are building capacity to make hundreds of millions of doses of their own or their partners’ vaccines.
The efforts are part of a larger rush, including at the White House, to line up funding for accelerated testing and expanded manufacturing capacity, all to make doses available in the U.S. starting as soon as this fall.
- 04/28/2020 – Pfizer could start distributing the vaccine on an emergency basis in the fall and receive approval for widespread distribution by year’s end; Moderna (the front runner) plans to have the third and final phase of testing could start in the fall. The company said it could seek FDA approval to sell the vaccine by year’s end, if it succeeds in testing. – So watch out for fall (September) and maybe it is a good idea to get options ready by Oct.
Race for Coronavirus Vaccine Accelerates as Pfizer Says U.S. Testing to Begin Next Week
The pharmaceutical firm joins various groups pushing to have vaccines ready for emergency use in the fall, though hurdles remain
The experimental vaccines still face a gauntlet of testing to make sure they work safely, which could derail efforts. Many promising drugs and vaccines wind up faltering during rounds of study. The average vaccine takes about 10.7 years to develop from its preclinical phase and has a market entry probability of 6%, according to a 2013 study published by PLOS ONE.
Adding to the potential obstacles, researchers say, is the fast-moving nature of the virus and measures to limit its spread. They have complicated efforts to set up some studies and find patients for research, delaying efforts and even closing some trials.
Yet research into a coronavirus vaccine has moved at a relatively rapid clip, infectious-disease experts say.
“I’m not aware of any vaccine that’s been developed after only a year to a year-and-a-half after identifying a pathogen. It usually takes years,” said Walter Orenstein, associate director of the Emory Vaccine Center in Atlanta. “People are moving very, very quickly with this.”
New York-based Pfizer is working on vaccine candidates with Germany’s BioNTech SE. The shots are based on an emerging gene-based technology known as messenger RNA, or mRNA, which carry instructions from DNA to the body’s cells to make certain proteins.
Testing of a vaccine, which has already started in Germany, could start in the U.S. as early as next week if health regulators sign off, Pfizer’s Mr. Bourla said. Results from the study could come as early as next month, he said.
If further testing also proves successful, Pfizer could start distributing the vaccine on an emergency basis in the fall and receive approval for widespread distribution by year’s end, Mr. Bourla said.
Moderna, which is also developing an mRNA vaccine, said it had asked the U.S. Food and Drug Administration to authorize the next phase of testing, a standard request before starting a new vaccine trial, and it could begin as early as May.
The study would test the experimental vaccine in about 600 healthy volunteers to see if it was safe and triggered the production of antibodies that could neutralize the coronavirus.
If the vaccine shows signs of working safely in the study, Moderna said the third and final phase of testing could start in the fall. The company said it could seek FDA approval to sell the vaccine by year’s end, if it succeeds in testing.
04/09/2020 – General knowledge of CV-19 vaccines
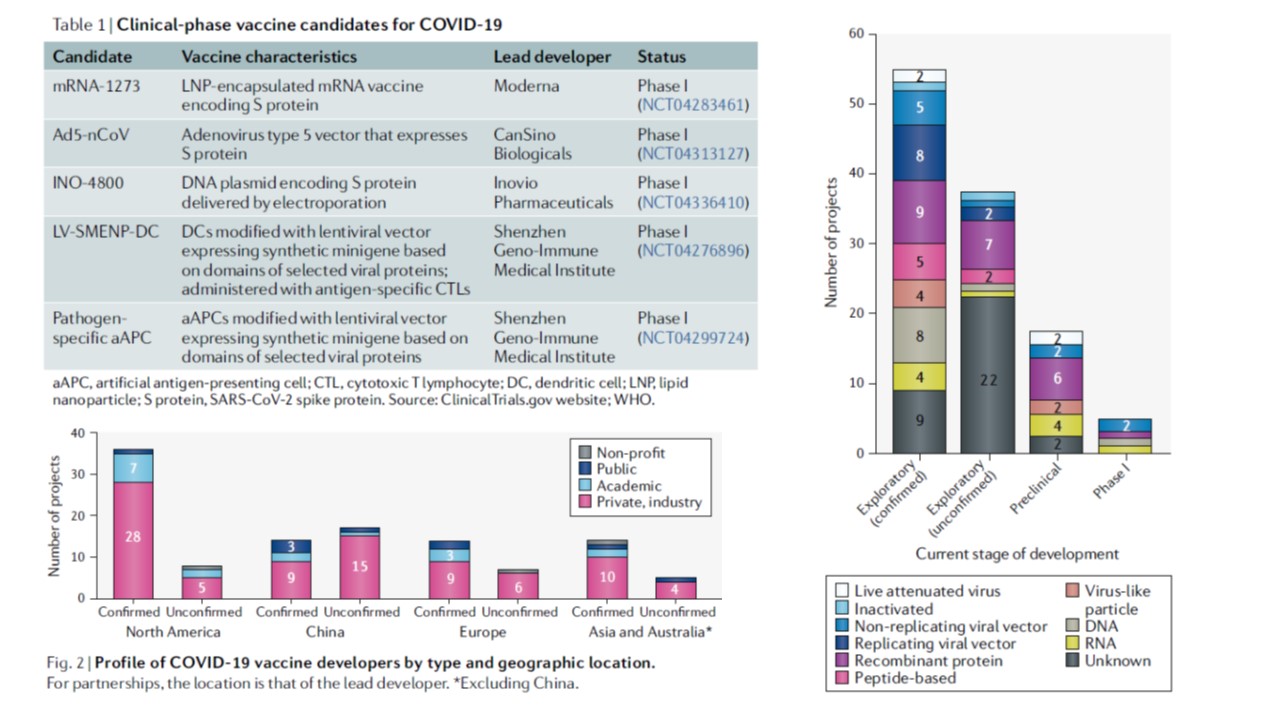
The COVID-19 vaccine development landscape (detailed paper in pdf d41573-020-00073-5)
novel-coronavirus-landscape-ncov
Moderna in wikipedia – is it a marvel or emperor’s new clothes?
